Re: full tank vs. empty tank for storage
Dang Phil, usually when I post something like post 36 above I never bother to look at the thread again cause no one ever pays any attention to them or replies to them! Now you?ve been all gracious and everything, and I feel guilty and have to back my crap up!

Pascoe has a mixture of gallons, meters, grams and ounces. Easiest thing to do to start is convert his 200 gallon tank to cubic feet. Since there are 7.48 gallons in 1 cubic foot, a 200 gallon tank = 200 / 7.48 = 26.78 cubic feet.
He claims this 26.78 cu_ft tank contains .81oz of water at 86F. Ok, I can live with that. We?ll determine what he used for Relative Humidity from the above two pieces of info, then we can compute the Dew Point. The Dew Point is the temp at which condensation starts taking place.
Since there are 16 oz in a lb, .81 oz of water equals .81/16 = .0506 lbs of water in the tank.
Next we need to know how many pounds of air are in the tank.
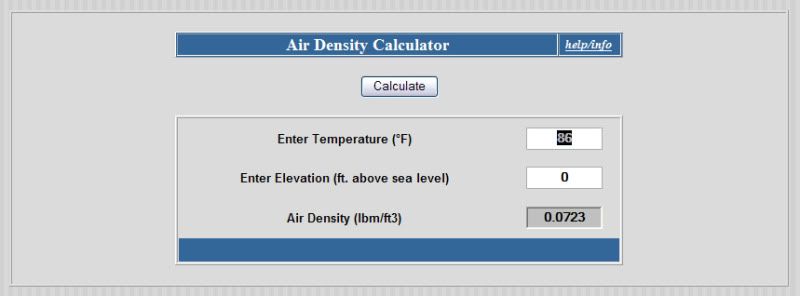
The air density calculator here will tell us how much a cubic foot of air weighs at the 86F temp:
http://www.denysschen.com/catalogue/density.asp
Since it weighs .0723 lb/cu_ft, and our tank is 26.78 cu_ft, that means we have .0723 x 26.78 = 1.933 lbs of air in the tank.
The Humidity Ratio is the amount of water in each pound of air. To find that, we divide the weight of water in the tank by the weight of the air: .0506 / 1.933 = .02618.
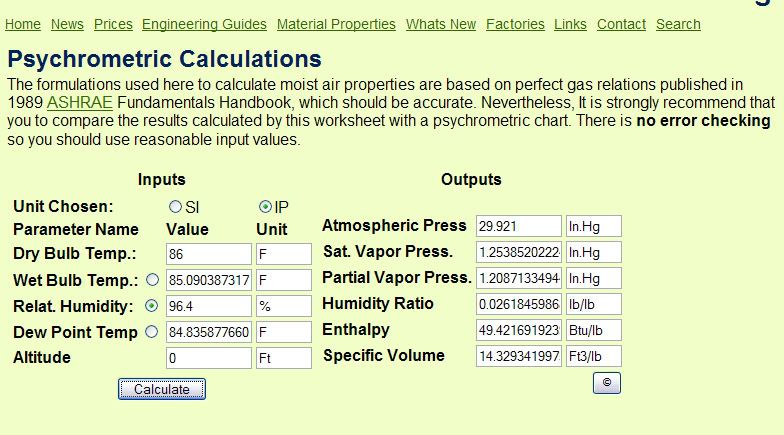
Now we can find the Relative Humidity he assumed, which will give us the Dew Point also. Easiest way is to adjust the Relative Humidity number in the psychometric calculator below till the Humidity Ratio equals the .02618 we calculated above. The only numbers I entered were the dry bulb temp of 86F, and then I played with the Relative Humidity entry until the Humidity Ratio equaled .02618 as we calculated above. Everything else was calculated by the psychrometric calculator webpage.
http://www.sugartech.co.za/psychro/index.php
What this means is that the ONLY way possible to have .81 oz of water in a 200 gallon tank at 86F is to have a Relative Humidity of 96.4%. Nothing wrong with that, he's free to pick any starting point he likes.
Now comes the interesting part. ?Dew Point? is the temperature at which condensation will start forming. Notice from the psychrometric calculator webpage what the Dew Point is at 86F and 94.6% relative humidity? It?s 84.84 degrees! This means that under his conditions of temperature and humidity, condensation will start forming on a surface that is 1.16 degrees colder than the air temperature! Hardly what I would call a ?much colder? surface!
Next, he states that at 50F the tank would contain .46 oz of water.
It?s real easy to do the same calcs as before. .46 / 16 = .0287 lb of water.
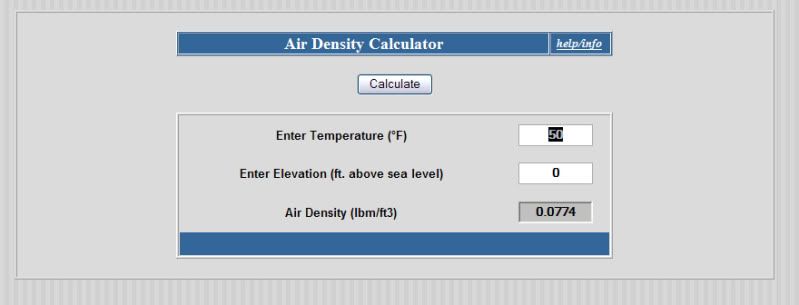
Air density at 50F is .0774 lb/cu_ft
So the tank has .0774 x 26.78 = 2.073 pounds of air in it.
.0287 / 2.073 = .0138 humidity ratio.
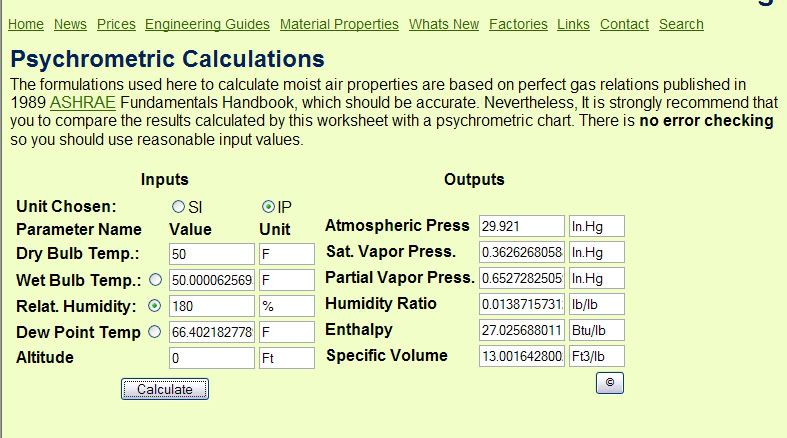
Now go to the psychrometric calculator, put in a dry bulb temp of 50F, and adjust the Relative Humidity until you get a Humidity Ratio of .0138.
As you can see, it would require a relative humidity of 180% to meet his condition that at 50F a 200 gallon tank contains .46 oz of water. Ain?t no way in this universe. Hopefully everyone here is aware that 100% relative humidity means that the air is completely saturated and can hold no more water. It?s possible to get briefly above 100% in a supersaturated state, but I very seriously doubt if many boaters have a Wilson Cloud Chamber and do nuclear physics experiments in their fuel tank ?.
From
http://en.wikipedia.org/wiki/Relative_humidity
The statement that relative humidity (RH%) can never be above 100%, while a fairly good guide, is not absolutely accurate, without a more sophisticated definition of humidity than the one given here. An arguable exception is the Wilson cloud chamber which uses, in nuclear physics experiments, an extremely brief state of "supersaturation" to accomplish its function.
To cover the points from post 36:
1) His stated condition of .46 oz of water at 50F in a 200 gallon tank is impossible.
2) He states that a surface must be ?much colder than air? for condensation to form at 86F with .81 oz of water in his 200 gallon tank. Reality is that he?s at a Relative Humidity of 96.4%, and it requires a temperature differential of 1.16F degrees for condensation to form under those conditions!
3) He claims that aluminum is second only to copper for heat transfer properties. Several things are better than copper for rapid heat transfer. At least he?s right in that aluminum is below copper. Graphite is 1950 Wm/k (we use it in rocket nozzles because it conducts the heat away so fast it doesn?t melt), diamond is 895, silver is 429, copper is 401, aluminum is 237.
http://en.wikipedia.org/wiki/List_of_thermal_conductivities
4) According to him, tank sweating is either ?rare? or ?likely to occur with boats in very cold waters when warm days are encountered?. All it takes is Relative Humidity and Temperature to tell when sweating will occur. At a high Relative Humidity, it takes a very small temperature drop for condensation to occur. Note: This is why it rains a lot in areas with high humidity. Raindrops require a microscopic piece of dust, smoke, etc to condense around (nuclei). A small drop in atmospheric temperature is enough to cause this condensation to begin, so it rains.
In summary, Pascoe calculates nothing but the amount of moisture in a closed system (he even gets that wrong 50% of the time).
A fuel tank is NOT a closed system. As simply as possible, to calculate the amount of condensation in a fuel tank would require determining the condensation rate by estimating the heat flow into the tank both from the atmosphere and from the condensation itself (btu/hr), then use the Enthalpy (btu/lb) from the psychrometric charts to determine the amount of water that is pulled out of the air in a given amount of time. That would give you lb/hr of water into the tank from condensation. You would also have to determine how much flow you have into the tank through the vent maintaining atmospheric equilibrium.
Good luck with that. I know some very smart people who have been working on it for over 25 years. It gets very critical at cryogenic (-238F) temperatures, and there?s a whole lot of stuff that goes into it:
http://wins.engr.wisc.edu/teaching/mpfBook/chapter9/node2.html